distribuidorUSA
Full Member
- Dec 11, 2012
- 148
- 34
- Detector(s) used
- Whites DFX.
- Primary Interest:
- All Treasure Hunting
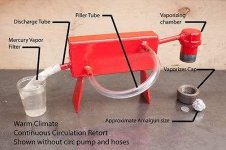
Hello everyone... I'm starting a small mining op in June.and Im thinking about using the Cianide Leach process to dissolve Gold and Silver from Ore that have -100 size particles.. I think I'll use Electrolysis to refine Gold and silver from the leach solution.. how do I separate the Gold from the Silver and any other contaminants like Copper or any other metal. I'm thinking about doing 50 gallons minimum at a time. I'm new on this and would like to know what materials to use..like what container to use,what amps to use for that size batch..I'm thinking about using a gold Cathode since I want to have only gold since the beginning. but what is the best material to use for the Anode..and what additives to use if any on the pregnant solution to make the process more efficient.. I will only have 12V batteries as a source for electricity.. any input will be greatly appreciated... and promise to post pictures of any Gold we recover..thank you in advance.. God bless.
Amazon Forum Fav 👍
Attachments
Last edited: