geolover
Full Member
- Dec 5, 2015
- 103
- 49
- Detector(s) used
- White's GMZ twin D-Gold Master (shrapnel & casing finder) Garrett pin pointer at
- Primary Interest:
- Prospecting
I could really use some help in identifying what other people were mining long ago. This mine runs through schist, it has a solid one foot thick stringer of red and white VUGGY quarts running through it. The stringer is like a big-fat plate (est 50'x100'x1') that sits about 20 degrees flat and into the hill. The quarts is crazy looking, it's white, red, and orange and very vuggy. I have sampled everything, especially the quartz and there are no signs of gold. I don't see any signs of gems. Maybe it was a mineral that they were chasing? A 100' tunnel is a lot of work for nothing?
I live near the Vail Lake area near Temecula, CA. There doesn't seem to be much gold activity around here. There's a couple of vertical shafts nearby. I found records for two prospect mines from the 70's , one for gold, other for feldspar? None of it makes any sense. All I can really count on is the schist and quartz.
Any recommendations on what I can look fo?? I added a few pics, hopefully they will make more sense of the geological setting. The pic of stringer is how it looks on the right hand side of shaft.
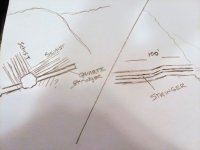



I live near the Vail Lake area near Temecula, CA. There doesn't seem to be much gold activity around here. There's a couple of vertical shafts nearby. I found records for two prospect mines from the 70's , one for gold, other for feldspar? None of it makes any sense. All I can really count on is the schist and quartz.
Any recommendations on what I can look fo?? I added a few pics, hopefully they will make more sense of the geological setting. The pic of stringer is how it looks on the right hand side of shaft.
















