cti4sw
Bronze Member
- Jul 2, 2012
- 1,555
- 919
- 🥇 Banner finds
- 1
- Detector(s) used
- Minelab Equinox 600, Garrett AT Pro, Pro Pointer
- Primary Interest:
- Relic Hunting
So, after making a first attempt (and having it not work so well), here is some info I gleaned from Google and personal experience.
I tried using a model railroading transformer and it actually barely put out enough current to cause a reaction, not to mention that the connections were not properly labeled so I had some aggravation trying to figure out the positive and negative leads.
Materials
1x plastic or glass container
1x power supply (6-24VDC / 2A)
1x box of sodium carbonate or sodium bicarbonate (soda)
2x steel alligator clips
2x lengths of wire
Xx metal item(s) with a lot of surface area
Tools
Wire strippers
Spoon measurement
Multimeter (optional)
Soldering iron (optional)
Solder (optional)
Preparation
1. You can get the wire lengths from an extra computer wall outlet cord by removing the outer black or gray rubber insulation.
2. Strip about 1½” off each end of the two lengths of wire.
3. Optional – Feed one end of a wire length through the alligator clip’s crimp. The clip itself may have a hole; if it does, you can feed the bare end of the wire through the hole and solder the wire in place. Do this for both wires; otherwise, twist the wire end securely, or crimp it in place.
4. Attach the other end of the wires to the power supply’s connections. Power supplies should be able to provide 6 to 24 volts of direct current (VDC) and about 2 amps. Car battery chargers can put out up to 10 amps with the required voltage and are relatively cheap; they may even come with their own leads and clips. Model train transformers do NOT make good power supplies.
5. Optional – If you are measuring current (amps), the multimeter must be connected in series (in line) with the other components to function properly. If you are measuring voltage, the multimeter can sit outside the circuit and probes can be used in parallel with the resistive load (the circuit components between nodes).
6. Dissolve about 1 cup of soda per 1 gallon of water in your container. Some sites may recommend a dash of table salt to increase conductivity, but keep in mind that salt water is very corrosive and too much salt can damage the item you’re trying to preserve. A salt electrolyte will also create chlorine gas which is very toxic.
Procedure
1. The best anode (+) material is steel (stainless is fine – do NOT use aluminum!) and has a large amount of surface area; old cookie sheets, sheet steel, steel pot/pan lids work perfectly. Clip the positive wire lead to the anode material and place the anode into the electrolyte solution. Do not place the anode’s alligator clip into the solution!
2. The cathode (-) is the item you want to clean. For best results, find a way to suspend the cathode item about 2” over the anode while keeping it fully immersed in the electrolyte. The cathode alligator clips may touch/be in the solution if necessary. If you opt to suspend the cathode, make sure your suspension materials are non-conductive (wood, plastic, PVC, etc).
3. Set the power supply to its highest setting and plug it in. Note the amperage on your multimeter; take care to not exceed the rating of your power supply. Once you turn on the power supply, do NOT touch either node nor the electrolyte solution! If you must make adjustments, unplug the power supply first.
4. If bubbles start to appear on and around the item you’re cleaning, you’ve successfully began the electrolysis process. If nothing happens, you should check the connections; you might have the cathode/anode connections switched. If not, perhaps the power supply is broken, or the output isn’t enough to initiate the process.
5. You should probably keep a close eye on the process to ensure that your item doesn’t get damaged by the electrolyte. The process should only remove oxidation from the cathode, but if there’s more oxidation than cathode, you may see some deterioration. Depending on the type of soda you use, the electrolyte may be more or less inherently corrosive.
When an item oxidizes (rusts), this is the chemical reaction occurring:
Fe3+ + O2- ---> Fe2O3
Finishing
1. Once the item is free of the oxidation, unplug the power supply and remove the item from the electrolyte solution. Freshly-cleaned items will be more susceptible to further oxidation so a preservative of some sort is necessary. For items used daily, such as tools, an oil-based substance or wax serves best. For relic preservation, a clear lacquer or wax is best. For old coins (where patina and tarnish are best left alone), you might use a soft preservative like olive oil.
Warnings
1. NEVER touch the electrolyte solution nor the nodes while the power supply is plugged in!
2. Do NOT use aluminum as the anode!
3. NEVER cover the bin! Released gases are in minute amounts, but gas buildup can have explosive results.
4. If the amperage on your power supply is too high, increase the distance between the nodes, reduce the amount of anode in the electrolyte solution, or increase the ratings on the power supply.
5. Avoid table salt (NaCl) in the electrolyte solution! It’s also redundant because sodium carbonate (NaCO3)¬ and sodium bicarbonate (NaHCO3) are salts themselves.
A Note About Electricity
It only takes 65 milliamps (0.065 A) to kill a person. The average car battery puts out 500 A.
Voltage rarely kills; current is the killer. You can think of voltage as the volume and current as the concentration.
Static electricity contains upwards of 3,000 V but a very low current.
Tasers put out over 100,000 V with low current.
A typical household wall outlet puts out 240 V with about 200 mA of current.
Cell phone battery chargers convert that to 5 V with about 700 mA of current.
Thanks to Rick’s Wood Shop Creations for his method and advice.
I tried using a model railroading transformer and it actually barely put out enough current to cause a reaction, not to mention that the connections were not properly labeled so I had some aggravation trying to figure out the positive and negative leads.
Materials
1x plastic or glass container
1x power supply (6-24VDC / 2A)
1x box of sodium carbonate or sodium bicarbonate (soda)
2x steel alligator clips
2x lengths of wire
Xx metal item(s) with a lot of surface area
Tools
Wire strippers
Spoon measurement
Multimeter (optional)
Soldering iron (optional)
Solder (optional)
Preparation
1. You can get the wire lengths from an extra computer wall outlet cord by removing the outer black or gray rubber insulation.
2. Strip about 1½” off each end of the two lengths of wire.
3. Optional – Feed one end of a wire length through the alligator clip’s crimp. The clip itself may have a hole; if it does, you can feed the bare end of the wire through the hole and solder the wire in place. Do this for both wires; otherwise, twist the wire end securely, or crimp it in place.
4. Attach the other end of the wires to the power supply’s connections. Power supplies should be able to provide 6 to 24 volts of direct current (VDC) and about 2 amps. Car battery chargers can put out up to 10 amps with the required voltage and are relatively cheap; they may even come with their own leads and clips. Model train transformers do NOT make good power supplies.
5. Optional – If you are measuring current (amps), the multimeter must be connected in series (in line) with the other components to function properly. If you are measuring voltage, the multimeter can sit outside the circuit and probes can be used in parallel with the resistive load (the circuit components between nodes).
6. Dissolve about 1 cup of soda per 1 gallon of water in your container. Some sites may recommend a dash of table salt to increase conductivity, but keep in mind that salt water is very corrosive and too much salt can damage the item you’re trying to preserve. A salt electrolyte will also create chlorine gas which is very toxic.
Procedure
1. The best anode (+) material is steel (stainless is fine – do NOT use aluminum!) and has a large amount of surface area; old cookie sheets, sheet steel, steel pot/pan lids work perfectly. Clip the positive wire lead to the anode material and place the anode into the electrolyte solution. Do not place the anode’s alligator clip into the solution!
2. The cathode (-) is the item you want to clean. For best results, find a way to suspend the cathode item about 2” over the anode while keeping it fully immersed in the electrolyte. The cathode alligator clips may touch/be in the solution if necessary. If you opt to suspend the cathode, make sure your suspension materials are non-conductive (wood, plastic, PVC, etc).
3. Set the power supply to its highest setting and plug it in. Note the amperage on your multimeter; take care to not exceed the rating of your power supply. Once you turn on the power supply, do NOT touch either node nor the electrolyte solution! If you must make adjustments, unplug the power supply first.
4. If bubbles start to appear on and around the item you’re cleaning, you’ve successfully began the electrolysis process. If nothing happens, you should check the connections; you might have the cathode/anode connections switched. If not, perhaps the power supply is broken, or the output isn’t enough to initiate the process.
5. You should probably keep a close eye on the process to ensure that your item doesn’t get damaged by the electrolyte. The process should only remove oxidation from the cathode, but if there’s more oxidation than cathode, you may see some deterioration. Depending on the type of soda you use, the electrolyte may be more or less inherently corrosive.
When an item oxidizes (rusts), this is the chemical reaction occurring:
Fe3+ + O2- ---> Fe2O3
Finishing
1. Once the item is free of the oxidation, unplug the power supply and remove the item from the electrolyte solution. Freshly-cleaned items will be more susceptible to further oxidation so a preservative of some sort is necessary. For items used daily, such as tools, an oil-based substance or wax serves best. For relic preservation, a clear lacquer or wax is best. For old coins (where patina and tarnish are best left alone), you might use a soft preservative like olive oil.
Warnings
1. NEVER touch the electrolyte solution nor the nodes while the power supply is plugged in!
2. Do NOT use aluminum as the anode!
3. NEVER cover the bin! Released gases are in minute amounts, but gas buildup can have explosive results.
4. If the amperage on your power supply is too high, increase the distance between the nodes, reduce the amount of anode in the electrolyte solution, or increase the ratings on the power supply.
5. Avoid table salt (NaCl) in the electrolyte solution! It’s also redundant because sodium carbonate (NaCO3)¬ and sodium bicarbonate (NaHCO3) are salts themselves.
A Note About Electricity
It only takes 65 milliamps (0.065 A) to kill a person. The average car battery puts out 500 A.
Voltage rarely kills; current is the killer. You can think of voltage as the volume and current as the concentration.
Static electricity contains upwards of 3,000 V but a very low current.
Tasers put out over 100,000 V with low current.
A typical household wall outlet puts out 240 V with about 200 mA of current.
Cell phone battery chargers convert that to 5 V with about 700 mA of current.
Thanks to Rick’s Wood Shop Creations for his method and advice.
Last edited:


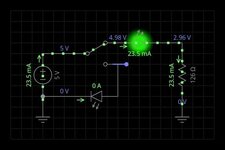
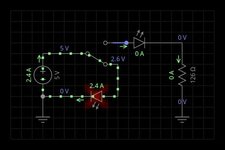
 as you can see from the pictures.
as you can see from the pictures.