zeeborg101
Newbie
- May 18, 2016
- 3
- 9
- Primary Interest:
- All Treasure Hunting
I picked up a couple of very old candlesticks last week.
I got them cheap at a thrift store, so figured what the heck.
I just got a fresh order of JSP silver and gold testing acid, and tried it.
Typically, when you put acid on base metals, they turn a green or bluish color (like the watch case pictures attached)
When I apply the silver test to the tarnished part (I believe is plate) it shows red flakes.
When I apply the gold test, NO reaction.
When I scrape a section off to clean it to fresh metal:
the silver test gives a whitish/gray residue and leaves a black mark.
the gold test creates a creamy light gray lump, and blackens the metal. (I've read that the creamy lump is silver nitrate?)
If I use acid on the shavings, they react violently, and give off a mustard colored smoke and then leaves a light gray 'ash'.
This has me very stumped.
Your thoughts?








I got them cheap at a thrift store, so figured what the heck.
I just got a fresh order of JSP silver and gold testing acid, and tried it.
Typically, when you put acid on base metals, they turn a green or bluish color (like the watch case pictures attached)
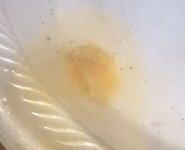
When I apply the silver test to the tarnished part (I believe is plate) it shows red flakes.
When I apply the gold test, NO reaction.
When I scrape a section off to clean it to fresh metal:
the silver test gives a whitish/gray residue and leaves a black mark.
the gold test creates a creamy light gray lump, and blackens the metal. (I've read that the creamy lump is silver nitrate?)
If I use acid on the shavings, they react violently, and give off a mustard colored smoke and then leaves a light gray 'ash'.
This has me very stumped.
Your thoughts?













