Green1
Silver Member
- Joined
- Mar 20, 2006
- Messages
- 3,930
- Reaction score
- 27
- Golden Thread
- 0
- Location
- Massachusetts
- Detector(s) used
- Mxt 6x10 coil Massachusetts
- #1
Thread Owner
Got this perfume bottle yesterday... The glass stopper is jammed in place.. I wanna get it out.. So i can clean it properly.. Any ideas.?
Thanks.. Rick
Thanks.. Rick




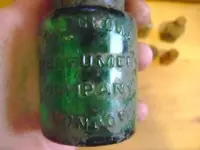

 Just a thought, be careful to warm the bottle up some before putting it in hot water so it won't crack
Just a thought, be careful to warm the bottle up some before putting it in hot water so it won't crack 






