I'm not sure they are inclusions. Agates are agates because the chalcedony is varicolored in interesting patterns. That doesn't mean anything is "included" in them.
The colors in rocks don't necessarily always come from having different minerals in them. A lot of the colors you see are due to light twisting through different densities of stone layers. Agates are composed of many many layers of deposited chalcedony. There are a lot of twists and turns for a photon of light to bounce it's way through.
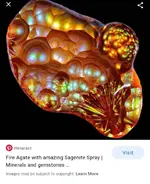
Colors of rocks can also come from inclusions of minute and very thin layers of water, organic material or oxides. The fire agates are in this class. Very very thin layers of iron oxides are interleaved with relatively pure silicon, this causes the light to bend as it traverses the stone and this effect is what creates the flash of colors you see. Those colors are not the color of the minute layers but the color the light is shifted to as it's path is lengthened or shortened as it traverses the different densities.
For these first two examples think of what happens when sunlight passes through a prism. The light is broken into it's many color components and organized according to the different lengths each ray has to pass through the glass (silicon) of the prism. Same principle with many rocks
Another way that stones exhibit color is through color centers. This takes place on an atomic level and has nothing to do with the inclusions but everything to do with the energy state of the atom itself. The quartz group has several good examples of how this works. Quartz crystals are clear right? - except when they are purple (amethyst), Orange (Citrine) or some other color. The purple and orange of Amethyst and Citrine are due to the energy state of their atoms. Leave either stone out in the bright sunlight long enough and the photons of sunlight hitting the individual atoms gradually, one by one, reduce the energy state of the atom and return the color centers back to the steady state of clear quartz.
With the lowered energy state from the sun exposure amethyst and citrine have become ordinary clear quartz without any change in chemical composition. You can also move the energy state of quartz color centers to a higher state with the addition of energy in the form of heat or radiation. Green quartz from some regions can be turned to amethyst with this method. It's commonly accepted in mineralogy that most, if not all amethyst quartz, get it's color from long term low level radiation exposure that tweaks the color centers. Most gem quality amethyst is treated with heat or radiation to further improve it's color.
These color centers are not a side note to mineral color. Most precious gems get their color from natural color centers. The colored iris in eyes come from color centers - not pigment. Color centers are everywhere. Color centers and their trapped atoms are being used in quantum computing to produce single photons on demand.
Now a further explanation why your Chalcedony doesn't have included Sagenite. Each pure mineral has one or more crystal habits. Habit is about the
exact way crystals are formed. If you know the crystal structure of a mineral you can identify the mineral by it's crystal habit.
If you look at the crystal habit of Sagenite rutile you will see that it's acicular. Acicular means the crystal is needle like. It's long, thin and very straight. If the crystal habit of the inclusions in a stone are not acicular you can bet good money they aren't Sagenite.
Wanna bet? I could use some easy money.

This is the shortened and simplified version of why some rocks have different colors. There is a lot more to it and some of the examples I gave aren't real accurate because I've simplified some things to make them easier to understand and to save wearing out my typing finger. I've been seriously studying this stuff for 52 years and I'm far from an expert. Now you have some terminology and concepts you can chase down and with a little luck you too can spend 52 years trying to understand rocks.