Sir Francis Bacon's procedure with using Mercury.
Under water? Why under water? I'd expect that immersion in water would leach out the very chemicals that were preserving the bodies. Were there experiments taking place with mercury/sulfur/arsenic/whatever in the water, or did you arbitrarily add the water? The Italian mummies were admittedly fascinating but I saw no mention of water. Are they stored in water?
Mercury is found in other places as well. It doesn't mean that a treasure is buried there, or that the mercury was not naturally occurring. More importantly, what compounds of mercury, sulfur, and arsenic were discovered? As all of these elements occur naturally in various compounds, were the compounds discovered found not to be naturally occurring?
Sir Francis Bacon was obsessed with the preservation of his manuscripts from a lifetime expenditure creating them.
He feared that some people of his era would plagiarize his works and call it theirs.
He set about experimenting with how to hide and still preserve these manuscripts.
Bacon had seen some ancient manuscripts which were preserved in Mercury, Sulfur, Arsenic, and wrapped in palm leaves and coated with tar and finally placed under water.
He claimed that these books were as good as when centuries ago they had been buried.
He set about experimenting to find the right formula.
From my limited logic on this subject, there are a number of obstacles that need to be overcome in order to mummify or preserve a document.
Humans, animals, rodents, pestilences, fungus, oxidation, mold, rot, moisture, cold, heat, dryness, humidity, etc.
I believe his final recipe was that a document wrapped in burlap, coated with an insoluble Mercury, insoluble Sulfur, a dash of Arsenic, placed in a wooden chest, coated once again with Mercury, Sulfur, and a pinch of Arsenic, wrapped in burlap and finally coating all this in a layer of tar and then placing this chest under water in a cavern sealed by an impregnable door of a 170 foot shaft containing dirt, wood, metal, with booby traps of water and Hydrogen Cyanide Gas, would accomplish his immortal time capsule.
“In 1934, before he began excavating, Hedden met a young man who had been working on the island for a month with a churn drill. Baker, as he was known, had made several deep holes near the Money Pit and told Hedden he had had no luck. But he had run across one peculiar thing. Once when the drill was pulled up he noticed specks of a silvery substance mixed with the clay on the point. It was free mercury.
(Mercury is almost never found in its metallic form in nature.)” Pen Leary, June 1953
"Mercury is used to purify gold from ore in a process called amalgamation. Miners and their families often inhale toxic mercury vapors through this process, and mercury can pollute homes and communities.[5] It can also contaminate the land and water where gold processing occurs."
In October 1779 the Royal Navy captured and sacked the Fortress San Fernando of Omoa, Honduras which became the Spanish’s Storage Facility for Silver shipments to Spain after Morro Castle, Cuba, was captured in 1762.
"The treasure found in the fort and on board two treasure ships was worth some two million dollars. Two hundred and fifty quintals of mercury were also found in the fort."
If Mercury was found at Fort San Fernando of Omoa would it not stand to reason that Mercury and Cyanide also were at Morro Castle, Cuba?
Mercury Vials were found on the island by Hedden.
The parchment paper "VI" contained Mercury on it.
Mercury was used for preserving wood.
Mercury can be found in elemental (liquid) form in nature, but this is not common.
Mercury does not react with water under normal conditions.
Mercury is made by roasting cinnabar in a furnace. The sulfide is oxidized to sulfur dioxide, leaving mercury behind.
Sulfur is insoluble in water.
Elemental sulfur does oxidize slowly to sulfurous acid, which in turn (through the action of sulfite) acts as a mild reducing and antibacterial agent.
Elemental sulfur is one of the oldest fungicides and pesticides.
Sulfur dioxide and various sulfites have been used for their antioxidant antibacterial preservative properties.




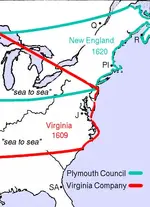
 Check your map. You're off about 600 miles!
Check your map. You're off about 600 miles!