Crispin
Silver Member
- Joined
- Jun 26, 2012
- Messages
- 3,584
- Reaction score
- 2,857
- Golden Thread
- 0
- Location
- Central Florida
- Detector(s) used
- Coinmaster Pro, Sand Shark
- Primary Interest:
- Other
First here is the coin...then the story.


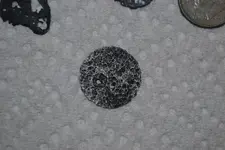

So I dug this up over a week ago at my antique spot. I thought is was a fried zincoln so I threw a bunch of them in vinegar and put it outside. Forgot about it until today. I brought the cup in and this is what I found (obviously the dime was not in there, I put that in for size reference:


So I'm thinking this is remarkably intact and rather odd. I bust out the oxidation test and put the all the coins from the test and the dime is a control, lots of bubbles on the unknown coin:

Okay, I'm convinced enough that it is silver to move on the acid test. I bring the zincolns as controls, control first:




Unknown coin second:



I snap a couple pictures to document and then rapidly pull it out as silver is starting to accumulate on the top, this is what it looks like back in the house (minus the control which completely disintegrated):

I cleaned it up as best I could back in the oxidation-reduction pan but I was afraid to keep cleaning because I was losing the coin. Got the wife to take the hi-res pics that are first posted. Anybody know what it could be? I realize there are no markings on it. Thanks for looking and thanks for any helpful comments. Click on the hi-res pictures to enlarge and then magnify for a better look.
PS: If anybody calls this an Aluminum fire nugget they have lost their mind.
PPS: I went through other finds from that area that I thought were zincolns and have them soaking in vinegar now. Will leave them for a week.




So I dug this up over a week ago at my antique spot. I thought is was a fried zincoln so I threw a bunch of them in vinegar and put it outside. Forgot about it until today. I brought the cup in and this is what I found (obviously the dime was not in there, I put that in for size reference:


So I'm thinking this is remarkably intact and rather odd. I bust out the oxidation test and put the all the coins from the test and the dime is a control, lots of bubbles on the unknown coin:

Okay, I'm convinced enough that it is silver to move on the acid test. I bring the zincolns as controls, control first:




Unknown coin second:



I snap a couple pictures to document and then rapidly pull it out as silver is starting to accumulate on the top, this is what it looks like back in the house (minus the control which completely disintegrated):

I cleaned it up as best I could back in the oxidation-reduction pan but I was afraid to keep cleaning because I was losing the coin. Got the wife to take the hi-res pics that are first posted. Anybody know what it could be? I realize there are no markings on it. Thanks for looking and thanks for any helpful comments. Click on the hi-res pictures to enlarge and then magnify for a better look.
PS: If anybody calls this an Aluminum fire nugget they have lost their mind.
PPS: I went through other finds from that area that I thought were zincolns and have them soaking in vinegar now. Will leave them for a week.
Last edited:






