cti4sw
Bronze Member
- Joined
- Jul 2, 2012
- Messages
- 1,555
- Reaction score
- 919
- Golden Thread
- 1
- Location
- Pennsylvania
- 🥇 Banner finds
- 1
- Detector(s) used
- Minelab Equinox 600, Garrett AT Pro, Pro Pointer
- Primary Interest:
- Relic Hunting
- #1
Thread Owner
So, I'd heard of electrolysis before but thought it was limited to hair removal  so once I read about some of you using it to clean artifacts I had to find out whether it was a simple process or not. Some definitions for you:
so once I read about some of you using it to clean artifacts I had to find out whether it was a simple process or not. Some definitions for you:
Oxidation: any surface destruction caused by a chemical reaction involving oxygen; rust and silver tarnish are two examples
Corrosion: any chemical reaction that erodes a substance over time; oxidation is one form of corrosion
Electrolysis: sending electricity through a liquid to separate oxidation and/or corrosion from metals
Cathode: the negative side of the battery
Anode: the positive side of the battery
Solvent: the liquid used to conduct the current and separate the oxidation/corrosion from the metal
It is simple. It could be messy, but it IS a simple process.
You could do this one of two ways.... either way includes a small 10-gallon fish tank, two steel rods, jumper cables, and some sort of power source. So, the 2 ways would be a car battery for big artifacts and a 9-volt for smaller artifacts. In any case, the juice from the power supply must "outweigh" (so to speak) or be equal to the energy transferred and lost in the reaction.
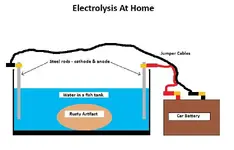
I would probably either clamp or use electrical tape to secure the alligator clips to the rim of the fish tank. Having never done this before, I have no idea how long it would take to completely free the artifact of the oxidation. I highly recommend you disconnect the anode BEFORE you touch the water!!! I cannot stress this enough. Voltage alone will not kill you; the average static shock is 3,000 volts and Tasers use about 100,000 volts. But 0.050 amps can kill you if it passes through your heart, and this is amplified by the water you'd be using.
I cannot stress this enough. Voltage alone will not kill you; the average static shock is 3,000 volts and Tasers use about 100,000 volts. But 0.050 amps can kill you if it passes through your heart, and this is amplified by the water you'd be using.
Anyone who tries this (or has done it before) please provide feedback...
~Jon
 so once I read about some of you using it to clean artifacts I had to find out whether it was a simple process or not. Some definitions for you:
so once I read about some of you using it to clean artifacts I had to find out whether it was a simple process or not. Some definitions for you:Oxidation: any surface destruction caused by a chemical reaction involving oxygen; rust and silver tarnish are two examples
Corrosion: any chemical reaction that erodes a substance over time; oxidation is one form of corrosion
Electrolysis: sending electricity through a liquid to separate oxidation and/or corrosion from metals
Cathode: the negative side of the battery
Anode: the positive side of the battery
Solvent: the liquid used to conduct the current and separate the oxidation/corrosion from the metal
It is simple. It could be messy, but it IS a simple process.
You could do this one of two ways.... either way includes a small 10-gallon fish tank, two steel rods, jumper cables, and some sort of power source. So, the 2 ways would be a car battery for big artifacts and a 9-volt for smaller artifacts. In any case, the juice from the power supply must "outweigh" (so to speak) or be equal to the energy transferred and lost in the reaction.


I would probably either clamp or use electrical tape to secure the alligator clips to the rim of the fish tank. Having never done this before, I have no idea how long it would take to completely free the artifact of the oxidation. I highly recommend you disconnect the anode BEFORE you touch the water!!!
 I cannot stress this enough. Voltage alone will not kill you; the average static shock is 3,000 volts and Tasers use about 100,000 volts. But 0.050 amps can kill you if it passes through your heart, and this is amplified by the water you'd be using.
I cannot stress this enough. Voltage alone will not kill you; the average static shock is 3,000 volts and Tasers use about 100,000 volts. But 0.050 amps can kill you if it passes through your heart, and this is amplified by the water you'd be using. Anyone who tries this (or has done it before) please provide feedback...
~Jon




