reptwar1
Sr. Member
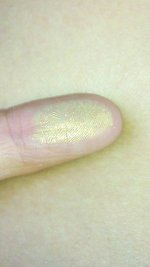
I live by the Arkansas river and dug out a crack in sidewalk, that is submerged when river floods. Literally took 3 minutes to collect this gold dust. It stuck to my dust pan, and I am wondering about ways to collect it. There are literally hundreds of more cracks for me to dig out. Thanks in advance 

Attachments
Upvote
0