Steve in PA
Gold Member
- Jul 5, 2010
- 9,579
- 14,092
- 🥇 Banner finds
- 4
- Detector(s) used
- Fisher F75, XP Deus, Equinox 600, Fisher 1270
- Primary Interest:
- All Treasure Hunting
I have posted a couple threads over the last two weeks about the rock that set my metal detector off in a plowed field. I had polished a spot on the rock with my bench grinder and tried to get an elemental analysis with our hand held PMI gun at work, although I am not trained to use it and it is much less accurate that our larger one that actually burns the surface of the material.
Today we had the technician back in the office that is trained to use this equipment and he took a reading using our Oxford PMI Master Pro Mobile Spectrometer.

Here is the spot that was tested before and after the burn


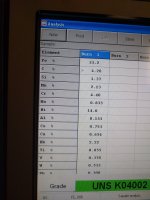
And here are the results: 33.2% Iron, 14.0% Nickel - I think there is still hope for this rock
Today we had the technician back in the office that is trained to use this equipment and he took a reading using our Oxford PMI Master Pro Mobile Spectrometer.

Here is the spot that was tested before and after the burn


And here are the results: 33.2% Iron, 14.0% Nickel - I think there is still hope for this rock









