GatorBoy
Gold Member
- May 28, 2012
- 14,716
- 6,149
- Primary Interest:
- All Treasure Hunting
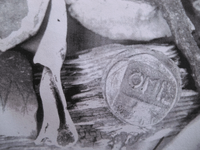
I found this quite a while back and have yet to see another example.
These three letters immediately make me think of the Mexico mint and assayer Diego Degoodoy.
The "o" is usually smaller on that mint mark as well.
I just thought I would post this for a look.
I'm also posting a few musket balls found close by as well as the marked olive jar rim from the same site.
Note...the lack of oxidation on some of the musket balls.
That should say enough about that.



These three letters immediately make me think of the Mexico mint and assayer Diego Degoodoy.
The "o" is usually smaller on that mint mark as well.
I just thought I would post this for a look.
I'm also posting a few musket balls found close by as well as the marked olive jar rim from the same site.
Note...the lack of oxidation on some of the musket balls.
That should say enough about that.



Last edited: